FDA GRAS Notification
Page Information

Contents
FDA GRAS Notification
 < FDA GRAS Notification >
< FDA GRAS Notification >
What is GRAS(Generally Recognized as Safe)?
- "GRAS" is an acronym for the phrase Generally Recognized As Safe. Under sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act (the Act), any substance that is intentionally added to food is a food additive, that is subject to premarket review and approval by FDA, unless the substance is generally recognized, among qualified experts, as having been adequately shown to be safe under the conditions of its intended use, or unless the use of the substance is otherwise excluded from the definition of a food additive.
- “GRAS Notice” means a submission that informs us of your view that a substance is not subject to the premarket approval requirements of the Federal Food, Drug, and Cosmetic Act based on your conclusion that the substance is GRAS under the conditions of its intended use in accordance with 21 CFR 170.30.
- Under sections 201(s) and 409 of the Act, and FDA's implementing regulations in 21 CFR 570.3 and 21 CFR 570.30, the use of a food additive may be GRAS either through scientific procedures or, for a substance used in food before 1958, through experience based on common use in food. Under 21 CFR 170.30(b), general recognition of safety through scientific procedures requires the same quantity and quality of scientific evidence as is required to obtain approval of the substance as a food additive. General recognition of safety through scientific procedures is based upon the application of generally available and accepted scientific data, information, or methods, which ordinarily are published, as well as the application of scientific principles, and may be corroborated by the application of unpublished scientific data, information, or methods. Also, under 21 CFR 170.30(c) and 170.3(f), general recognition of safety through experience based on common use in foods requires a substantial history of consumption for food use by a significant number of consumers.
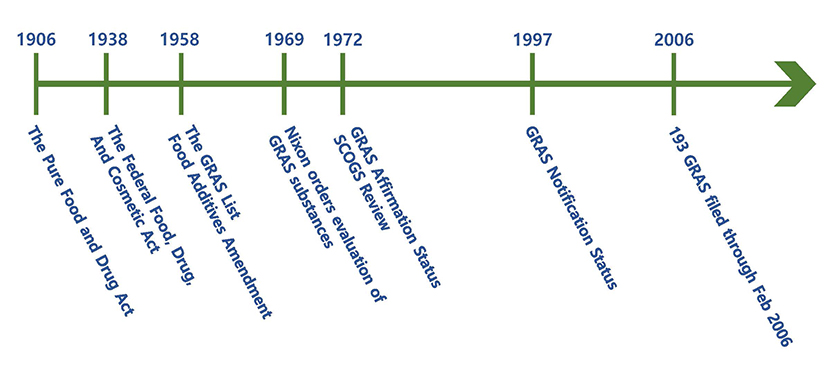 < History of GRAS (Reference: FDA) >
< History of GRAS (Reference: FDA) >
History of GRAS
The picture above is a timeline from the passage of the Pure Food and Drug Act in 1906.
-
Implementation of GRAS
- • In 1958, Food Additives Amendment was enacted because the Congress recognized that many food substances would not require a formal premarket review by FDA to assure their safety. It was either because their safety had been established by a long history of use in food or by virtue of the nature of the substances, their conditions of use, and the information generally available to scientists. Accordingly, GRAS and certain substances(ex. color additives) were exempted from existing food additives. In December 1958, FDA published a list of GRAS substances and incorporated the list in Title 21 of the Code of Federal Regulations. The current list appears in 21 CFR Parts 182, 184, and 186.
- • Many substances that were considered GRAS by the food industry were not included in FDA's 1958 GRAS list. After that, many manufacturers sent opinion letters to the FDA requesting that FDA official provide an informal opinion on the GRAS status of use of the substance, however, was revoked in 1970.
-
GRAS review begins
- • On October 30th, 1969, when the FDA removed cyclamate from the GRAS list due to safety concerns, then-President Nixon directed FDA to make a critical evaluation of the safety of GRAS food substances. During the 1970s and 1980s, the LSRO(Life Sciences Research Office) selected the qualified scientists, SCOGS(Select Committee on GRAS Substances), as consultants to review and evaluate the available information on each of the GRAS substances. FDA also made tentative reports from SCOGS available to the public and provided opportunity for the public to appear before the Select Committee at a public hearing. SCOGS considered the data, information, and views presented at the hearing in developing its final reports.
- • By 1982, SCOGS had submitted opinions to the FDA on the health aspects of more than 400 substances, and the FDA had reviewed many presumed GRAS substances.
-
Filing and Implementing of GRAS affirmation review petitions
- • In 1972, FDA established the GRAS affirmation rule(21 CFR 170.35). The rulemaking included a mechanism (the GRAS affirmation petition process) through which individuals could petition FDA to review GRAS status that is not considered part of FDA’s GRAS review.
-
Implementation of GRAS Notification
- • In April 1997, the FDA proposed to establish a notification procedure whereby a person may inform FDA of a determination that the use of a substance is GRAS. Accordingly, to ease regulatory procedures, FDA had announced a proposed regulation to change the GRAS prior approval system to a GRAS notification system and to evaluate the data submitted by notifier.
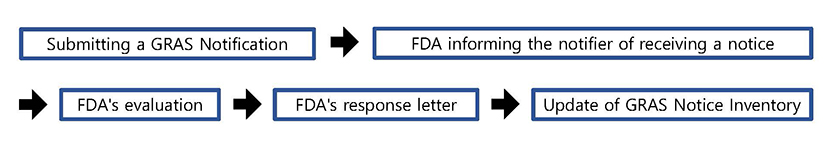 < Process of GRAS Notification Program >
< Process of GRAS Notification Program >
Process of GRAS Notification Program
- The GRAS notification program provides a voluntary mechanism whereby a person may inform FDA of a determination that the use of a substance is GRAS, rather than petition FDA to affirm that the use of a substance is GRAS.
-
FDA encourages individuals to thoroughly review its GRAS notification submission procedures prior to sending a notification to the Agency. Additionally, a notifier may request a pre-submission meeting with FDA to discuss issues that may be relevant to the submission of the notifier's GRAS notice.
- • When you submit your GRAS notice, you may do so either in an electronic format that is accessible for our evaluation or on paper. If you send your GRAS notice on paper, a single paper copy is sufficient.
- Within 30 days of receiving a notice FDA will inform the notifier in writing of the date on which the notice was received. FDA then evaluates whether the submitted notice provides a sufficient basis for a GRAS determination and whether information in the notice, or otherwise available to FDA, raises issues that lead the agency to question whether use of the substance is GRAS. On occasion, FDA will consult with other agencies; for example, when the notice includes use in meat and poultry products, FDA consults with the Food Safety and Inspection Service of the U.S. Department of Agriculture.
-
Following this evaluation FDA responds to the notifier with one of three types of letters :
- ① The first type of letter states that FDA does not question the basis for the notifier's GRAS determination. This type of letter may also note, among other things, potentially pertinent issues related to labeling, the substance's use in certain foods, and requirements for a color additive.
- ② In the second type of letter, the agency concludes that the notice does not provide a sufficient basis for a GRAS determination (e.g., because the notice does not include appropriate data and information, or because the available data and information raise questions about the safety of the notified substance).
- ③ The third type of letter states that the agency has, at the notifier's request, ceased to evaluate the GRAS notice.
* FDA updates the result of GRAS Notification on the web page titled 'Summary of All GRAS Notices.
https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices
 < Documents for GRAS Notification Program >
< Documents for GRAS Notification Program >
Documents for GRAS Notification Program
Followings are the required documents needed when notifying GRAS with the form FDA 3667 :
- • Introductory information about the submission
- • Information about the notifier (and any attorney or agent acting on behalf of the notifier)
- • General administrative information
- • Intended use
- • Identity
- • Method of manufacture
- • Specifications
- • Dietary exposure
- • Self-limiting levels of use
- • Common use in food before 1958 (if applicable)
- • Comprehensive discussion of the basis for the GRAS determination
- • Bibliography
- • Signature
- PreviousISO/SAE 21434:2021 – Road vehicles – Cybersecurity engineering 23.12.04
- NextISO 37301:2021 Compliance Management Systems 23.10.16
Comment list
There are no registered comments.

